|
14.08.2017 14:00:00
|
DarioHealth Reports Second Quarter 2017 Results
CAESAREA, Israel, August 14, 2017 /PRNewswire/ -- DarioHealth Corp. (NASDAQ: DRIO), a leading global digital health company with mobile health and big data solutions, today reported financial and operational results for the three-month period ended June 30, 2017.

Second Quarter Highlights
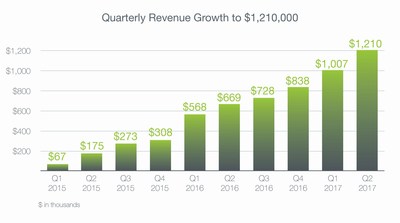
- Record revenues of $1,210,000, increased 81% compared to the second quarter of 2016
- Sequential quarterly revenue growth of 20% over the first quarter of 2017
- 75% of quarterly revenues derived from test strips and other consumables
- Gross profit of 29.8% as a result of increase in quantities sold and higher average sale prices together with an increase in consumable sales
- More Than 8,000 Dario™ Blood Glucose Monitoring System devices sold in the U.S.; reaching cumulative sales of more than 33,000 devices in the U.S.
- Cash burn decreased by $338,000 or 11% compared to the first quarter of 2017
DarioHealth expects a continued reduction in its cash burn over the next several quarters as it penetrates and acquires new users in the Android universe.
In addition, DarioHealth expects its business-to-consumer (B2C) strategy to be more efficient by increasing its customer value and reducing its cost per customer acquisition. Having recently achieved key milestones, such as FDA clearance for certain leading Android devices in the U.S. and the signing of additional third-party insurance coverage agreements, management remains confident in its B2C strategy and its ability to gain a wider audience.
On the business-to-business (B2B2C) side, DarioHealth expects to partner with additional players that will be able to introduce the DarioHealth platform to payers and the self-insured market in the U.S., providing their users a fully connected and real-time solution combined with coaching services. As the market is transforming to value-based solutions where payers are looking to buy a fully integrated solution, DarioHealth's platform is well positioned to address this need. DarioHealth has already signed its first agreement with CCS connect to provide payers a joint solution of platform and coaching services.
Erez Raphael, Chairman and CEO of DarioHealth, commented, "We are pleased with the continued sequential quarterly growth and traction we are gaining in the U.S. It is encouraging to see our margins improve because of our growing customer base of loyal users.
"With our recent Android clearance, we've greatly expanded our platform availability in the United States. This development, coupled with our third-party insurance coverage expansion, opens significant revenue opportunities on the B2C front that can be scaled up and help lower the burn rate. In parallel, the recent Android clearance by the FDA also opens a whole new revenue stream from B2B2C opportunities. Digital health solutions give payers an opportunity to reduce costs while improving clinical outcomes, and we believe that the DarioHealth connected model addresses this need and enables the Company to make significant gains."
Financial Results for the Three Months Ended June 30, 2017:
Revenue for the three months ended June 30, 2017 was $1,210,000, an 81% increase from $669,000 for the three months ended June 30, 2016, and a 20% increase sequentially from the first quarter of 2017. The increase in revenues is mainly a result of continued market penetration into the United States and Australia.
Revenues were derived mainly from the sales of Dario™'s components, including the Smart Meter itself, through direct sales to consumers located mainly in the United States and Australia, through our on-line store and through distributors.
Gross profit of $360,000 was recorded in the second quarter ended June 30, 2017, an increase of $517,000 compared to a gross loss of $157,000 in the second quarter of 2016. This represented a record gross profit of 29.8% as a result of increase in quantities sold and higher average sale prices, together with an increase in consumable sales.
Research and development expenses increased by $0.7 million, or 127%, to $1.2 million for the three months ended June 30, 2017, compared to $0.5 million for the three months ended June 30, 2016. This increase was mainly due to increases in salaries, stock based compensation and costs associated with clinical trials.
Sales and marketing expenses increased by $1.1 million, or 93%, to $2.2 million for the three months ended June 30, 2017 compared to $1.1 million for the three months ended June 30, 2016. These increases were mainly due to growth of our sales and marketing activities in the United States and Australia, an increase in costs of online marketing campaigns, the cost related to marketing consultants and the costs associated with subcontractors, employee payroll and stock based compensation.
Operating loss for the three months ended June 30, 2017 increased by $1.5 million to $4.1 million, compared to a $2.6 million operating loss in the three months ended June 30, 2016. This increase is mainly due to the increase in our operating expenses partially offset by the increase in the gross profit.
Net loss was essentially flat at $4.1 million for the three months ended June 30, 2017 compared to a loss of $4.1 million for the three months ended June 30, 2016.
As of June 30, 2017, cash and cash equivalents totaled $3.9 million.
Financial Results for the Six Months Ended June 30, 2017:
Revenue for the six months ended June 30, 2017 was $2,217,000, a 79% increase from $1,237,000 for the six months ended June 30, 2016.
Gross profit of $466,000 was recorded for the six months ended June 30, 2017, an increase of $725,000 compared to a gross loss of $259,000 for the six months ended June 30, 2016. This represented a record gross profit of 21.0% as a result of increase in quantities sold and higher average sale prices, together with an increase in consumable sales.
Research and development expenses increased by $0.7 million, or 80%, to $1.6 million for the six months ended June 30, 2017 compared to $0.9 million for the six months ended June 30, 2016.
Sales and marketing expenses increased by $2.4 million, or 143%, to $4.0 million for the six months ended June 30, 2017 compared to $1.6 million for the six months ended June 30, 2016.
Operating loss for the six months ended June 30, 2017 increased by $3.8 million to $8.3 million, compared to a $4.5 million operating loss for the six months ended June 30, 2016.
Financial income of $7.5 million was recorded in the six months ended June 30, 2017, compared to a financial expense of $1.9 million in the six months ended June 30, 2016. This change was mainly due to reversing the warrant revaluation expense recorded in the fourth quarter of 2016, due to a price protection feature included in warrants issued to investors in March and August 2016. This price protection feature expired on March 8, 2017, and as a result we cancelled the liability related to these warrants by recording financing income of $7.4 million.
Net loss was $850,000 for the six months ended June 30, 2017 compared to a loss of $6.4 million for the six months ended June 30, 2016. The decrease in net loss for the six months ended June 30, 2017 compared to the six months ended June 30, 2016 was mainly due to our financial income related to revaluation of warrants.
DarioHealth will host a second quarter 2017 conference call today at 9:00 AM ET.
Conference Call Details:
Date: Monday, August 14, 2017
Time: 9:00 AM ET
Dial-in Number: (888) 567-1603
International Dial-in Number: (862) 255-5347
Webcast: http://www.investorcalendar.com/event/19824
Participants are recommended to dial-in approximately 10 minutes prior to the start of the event. A replay of the call will be available approximately two hours after completion through November 14, 2017. To listen to the replay, dial (877) 481-4010 (domestic) or (919) 882-2331 (international) and use replay ID 19824. The webcast replay will be available through November 14, 2017.
About DarioHealth Corp.
DarioHealth Corp. (NASDAQ: DRIO) is a leading global digital health company serving tens of thousands of users with dynamic mobile health solutions. We believe people deserve the best tools to manage their treatment, and harnessing big data, we have developed a unique way for our users to analyze and personalize their diabetes management. With our smart diabetes solution, users have direct access to track and monitor all facets of diabetes, without having the disease slow them down. The acclaimed Dario™ Blood Glucose Monitoring System all-in-one blood glucose meter and native smartphone app gives users an unrivaled method for self-diabetes management. DarioHealth is headquartered in Caesarea, Israel with a regional office in Burlington, Massachusetts. For more information, visit http://mydario.investorroom.com/.
Cautionary Note Regarding Forward-Looking Statements
This news release and the statements of representatives and partners of DarioHealth Corp. (the "Company") related thereto contain or may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Statements that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as "plan," "project," "potential," "seek," "may," "will," "expect," "believe," "anticipate," "intend," "could," "estimate," or "continue" are intended to identify forward-looking statements. For example, the Company is using forward-looking statements in this press release when it describes expected reduction in cash burn, expectations for business to consumer and business to business channels, and the impact of Android clearance.. Readers are cautioned that certain important factors may affect the Company's actual results and could cause such results to differ materially from any forward-looking statements that may be made in this news release. Factors that may affect the Company's results include, but are not limited to, regulatory approvals, product demand, market acceptance, impact of competitive products and prices, product development, commercialization or technological difficulties, the success or failure of negotiations and trade, legal, social and economic risks, and the risks associated with the adequacy of existing cash resources. Additional factors that could cause or contribute to differences between the Company's actual results and forward-looking statements include, but are not limited to, those risks discussed in the Company's filings with the U.S. Securities and Exchange Commission. Readers are cautioned that actual results (including, without limitation, the timing for and results of the Company's commercial and regulatory plans for Dario™ as described herein) may differ significantly from those set forth in the forward-looking statements. The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
CONSOLIDATED BALANCE SHEETS | |||||||
U.S. dollars in thousands | |||||||
June 30, | December 31, | ||||||
2017 | 2016 | ||||||
Unaudited | |||||||
ASSETS | |||||||
CURRENT ASSETS: | |||||||
Cash and cash equivalents | $ 3,898 | $ 1,093 | |||||
Short-term bank deposits | 243 | 225 | |||||
Trade Receivables | 372 | 226 | |||||
Inventories | 717 | 888 | |||||
Other accounts receivable and prepaid expenses | 632 | 504 | |||||
Total current assets | 5,862 | 2,936 | |||||
LEASE DEPOSITS | 42 | 35 | |||||
PROPERTY AND EQUIPMENT, NET | 831 | 901 | |||||
Total assets | $ 6,735 | $ 3,872 | |||||
CONSOLIDATED BALANCE SHEETS | ||||||
U.S. dollars in thousands (except stock and stock data) | ||||||
June 30, | December 31, | |||||
2017 | 2016 | |||||
Unaudited | ||||||
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIENCY) | ||||||
CURRENT LIABILITIES: | ||||||
Trade payables | $ 985 | $ 1,812 | ||||
Other accounts payable and accrued expenses | 1,794 | 1,113 | ||||
Total current liabilities | 2,779 | 2,925 | ||||
LIABILITY RELATED TO WARRANTS | 3 | 7,488 | ||||
STOCKHOLDERS' EQUITY (DEFICIENCY) | ||||||
Common Stock of $0.0001 par value - Authorized: 160,000,000 shares at June 30, 2017 | 6 | 6 | ||||
Preferred Stock of $0.0001 par value - Authorized: 5,000,000 shares at June 30, 2017 | - | - | ||||
Additional paid-in capital | 59,757 | 48,413 | ||||
Accumulated deficit | (55,810) | (54,960) | ||||
Total stockholders' equity (deficiency) | 3,953 | (6,541) | ||||
Total liabilities and stockholders' equity (deficiency) | $ 6,735 | $ 3,872 | ||||
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS | |||||||||
U.S. dollars in thousands (except stock and stock data) | |||||||||
Three months ended June 30 | Six months ended June 30 | ||||||||
2017 | 2016 | 2017 | 2016 | ||||||
Unaudited | Unaudited | ||||||||
Revenues | $ 1,210 | $ 669 | $ 2,217 | $ 1,237 | |||||
Cost of revenues | 850 | 826 | 1,751 | 1,496 | |||||
Gross profit (loss) | 360 | (157) | 466 | (259) | |||||
Operating expenses: | |||||||||
Research and development | $ 1,184 | $ 521 | $ 1,653 | $ 918 | |||||
Sales and marketing | 2,205 | 1,142 | 4,030 | 1,661 | |||||
General and administrative | 1,089 | 803 | 3,106 | 1,708 | |||||
Total operating expenses | 4,478 | 2,466 | 8,789 | 4,287 | |||||
Operating loss | (4,118) | (2,623) | (8,323) | (4,546) | |||||
Financial income (expenses), net: | |||||||||
Revaluation of warrants | 25 | (1,428) | 7,485 | (1,850) | |||||
Other financial (expense) income, net | 1 | (6) | (12) | (19) | |||||
Total financial income (expenses), net | 26 | (1,434) | 7,473 | (1,869) | |||||
Net loss | $ (4,092) | $ (4,057) | $ (850) | $ (6,415) | |||||
Deemed dividend related to Series A Preferred Stock exchange agreement | $ - | $ - | $ - | $ 455 | |||||
Net loss attributable to holders of Common Stock | $ (4,092) | $ (4,057) | $ (850) | $ (6,870) | |||||
Net loss per share | |||||||||
Basic and diluted loss per share | $ (0.43) | $ (0.73) | $ (0.10) | $ (1.48) | |||||
Weighted average number of Common Stock used in computing basic and diluted net loss per share | 9,521,730 | 5,587,800 | 8,377,667 | 4,644,495 | |||||
CONSOLIDATED STATEMENTS OF CASH FLOWS | ||||
U.S. dollars in thousands | ||||
Six months ended June 30, | ||||
2017 | 2016 | |||
Unaudited | ||||
Cash flows from operating activities: | ||||
Net loss | $ (850) | $ (6,415) | ||
Adjustments required to reconcile net loss to net cash used in operating activities: | ||||
Stock-based compensation and Common Stock to service providers | 2,441 | 668 | ||
Depreciation | 101 | 191 | ||
Increase in trade receivables | (146) | - | ||
Decrease (increase) in accounts receivables and prepaid expenses | (146) | 84 | ||
Decrease (increase) in inventories | 171 | (410) | ||
Decrease (increase) in trade payables | (827) | 27 | ||
Decrease in deferred revenues | - | (31) | ||
Increase in other accounts payable and accrued expenses | 915 | 238 | ||
Change in fair value of warrants to purchase shares of Common Stock | (7,485) | 1,850 | ||
Net cash used in operating activities | (5,826) | (3,798) | ||
Cash flows from investing activities: | ||||
Maturity of (investment in) lease deposits | (7) | 1 | ||
Purchase of property and equipment | (31) | (246) | ||
Net cash used in investing activities | (38) | (245) | ||
Cash flows from financing activities: | ||||
Proceeds from issuance of Common Stock and warrants, net of issuance cost | 8,669 | 7,538 | ||
Proceeds from exercise of options and warrants | - | 210 | ||
Net cash provided by financing activities | 8,669 | 7,748 | ||
Increase in cash and cash equivalents | 2,805 | 3,705 | ||
Cash and cash equivalents at the beginning of the period | 1,093 | 2,671 | ||
Cash and cash equivalents at the end of the period | $ 3,898 | $ 6,376 | ||
Non-cash investing and financing activities: | ||||
Conversion of Series A Preferred Stock to Common Stock | $ - | $ 2,277 | ||
Payment for directors and employees under Stock for Cash Program | $ 183 | $ 102 | ||
DarioHealth Corporate and Media Contact
Shmuel Herschberg
Marketing Director
shmuel@mydario.com
1-914-775-5548
DarioHealth Investor Relations Contact
Hayden IR
Stephen Hart
DRIO@HaydenIR.com
1-917-658-7878
View original content with multimedia:http://www.prnewswire.com/news-releases/dariohealth-reports-second-quarter-2017-results-300503657.html
SOURCE DarioHealth Corp.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu DarioHealth Corpmehr Nachrichten
| Keine Nachrichten verfügbar. |