|
04.05.2021 22:55:00
|
Paragon 28, Inc. Receives FDA Clearance and Successfully Completes First MAVEN™ Patient-Specific Instrumentation Surgery
ENGLWOOD, Colo., May 4, 2021 /PRNewswire/ -- Paragon 28, Inc. unveils the highly anticipated MAVEN™ PSI System, a platform technology thoughtfully designed to expedite accurate positioning of the APEX 3D™ Total Ankle Replacement System.

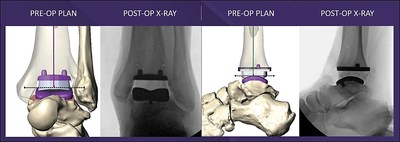
MAVEN PSI advances patient-specific technologies with anatomical design features promoting alignment accuracy, stability, and ideal implant placement. MAVEN PSI is compatible with both weight-bearing CT and traditional CT scanning technologies and features one continuous scan from the knee through the base of the foot to capture biomechanically accurate data. The combination of patient-specific CT information, contoured guides, and APEX 3D implant designs provide the surgeon with exceptional technology streamlined to optimize patient outcomes.
Design Surgeon Dr. Jeffrey Christensen performed the first MAVEN surgery in Seattle, Washington last week and stated:
"MAVEN PSI technology is in a class of its own. The System introduces a perfect blend of performance and reliability. Most importantly, the System allowed for excellent pre-operative visualization of the patient's anatomic structures, established precise component alignment, and helped to determine accurate implant size selection and placement which is critical for long term survivorship.1"
As a system, MAVEN PSI includes three unique anatomically contoured alignment guides, bone models, and a customized pre-operative plan. The System features the first and only Tibial AP Positioning Spacer on the market, developed to simplify the time-consuming sizing evaluation process of the tibial component and establish optimal tibial bone coverage. MAVEN PSI also allows for refined intra-operative positioning and micro adjustments if necessary. Learn more about the MAVEN PSI System here.
About Paragon 28, Inc.
Paragon 28, Inc. exclusively serves the foot and ankle surgical community and is dedicated to enhancing patient outcomes by advancing the science and research behind innovative technologies. The addition of MAVEN PSI bolsters Paragon 28's Precision Ankle Solutions product offering, which includes the APEX 3D™ Total Ankle Replacement System, Gorilla® Ankle Fracture Plating System, Silverback® Ankle Fusion Plating System, and Phantom™ TTC Fusion Nail Systems. With this comprehensive portfolio, Paragon 28 provides its customers innovative ankle solutions for trauma, arthritis, and limb salvage.
For more information, please visit www.apexankle.com or contact apex3d@paragon28.com
Reference
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/paragon-28-inc-receives-fda-clearance-and-successfully-completes-first-maven-patient-specific-instrumentation-surgery-301283815.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/paragon-28-inc-receives-fda-clearance-and-successfully-completes-first-maven-patient-specific-instrumentation-surgery-301283815.html
SOURCE Paragon 28, Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!