|
16.05.2018 14:00:00
|
Photocure Announces Blue Light Cystoscopy with Cysview® Now Available for Surveillance of Bladder Cancer
OSLO, Norway, May 16, 2018 /PRNewswire/ -- Photocure ASA (OSE: PHO), May 16, 2018, announced today the launch of Blue Light Cystoscopy (BLC™) with Cysview for a new indication of surveillance cystoscopy of bladder cancer.

The new FDA approved indication was a result of a large Phase 3 study using blue light rigid and flexible cystoscopes from KARL STORZ Endoscopy of America Inc., which showed that BLC with Cysview significantly (p<0.0001) improves detection of patients with recurrent bladder cancer. Click here for the Prescribing Information.
"We are pleased that we have commenced the first commercial sale of Cysview for surveillance cystoscopy. This launch into the flexible cystoscopy segment is an important milestone for patients with bladder cancer and the urology community. The availability of Blue Light Cystoscopy with Cysview, for rigid and flexible cystoscopy means that Cysview can now be used for both Bladder Cancer surgery and follow-up cystoscopy, allowing physicians to detect patients with the disease earlier and manage it more appropriately," says Kjetil Hestdal, MD, PhD, President and CEO, Photocure ASA. "I would like to thank all the investigators, our partner KARL STORZ Endoscopy of America Inc. and the patients who made the approval of this new indication possible."
Both companies are exhibiting at the 2018 American Urological Association Annual meeting in San Francisco, May 18 to 21, Photocure on booth 1825.
About Bladder Cancer
Bladder cancer is the fifth most commonly diagnosed cancer in the US and is the fourth most common cancer found in men in the US1, 2, 3. It is estimated that in 2018 there will be 81,190 new cases of bladder cancer will occur along with 17,240 deaths due to bladder cancer. Risk factors for bladder cancer include advancing age, cigarette smoking, occupational exposure to dyes, tar, rubber and solvent, chronic bladder irritation and infections, and prior diagnosis of bladder cancer. Bladder cancer is one of the most expensive cancers to manage, accounting for approximately $3.7 billion in direct costs each year.4,5
Bladder cancer is classified into two types, non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC), depending on the depth of invasion in the bladder wall.2 NMIBC is still in the inner layer of cells. These cancers are the most common (75%) of all BC cases and include the subtypes Ta, carcinoma in situ (CIS) and T1 lesions. MIBC is when the cancer has grown into deeper layers of the bladder wall. These cancers, including subtypes T2, T3 and T4, are more likely to spread and are harder to treat.2
About Hexvix®/Cysview®
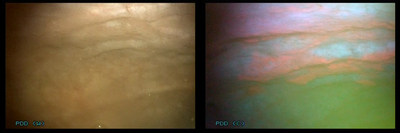
Hexvix®/Cysview® (hexaminolevulinate hydro-chloride), is an optical imaging agent used in the diagnosis and management of non-muscle-invasive bladder cancer. It is designed to selectively target malignant cells in the bladder and induce fluorescence during a cystoscopic procedure using a blue light enabled cystoscope. Using Cysview® as an adjunct to standard white light cystoscopy enables the urologist to better detect and remove lesions, leading to a reduced risk of recurrence.
Hexvix® is the tradename in Europe, Cysview® in US and Canada. Hexvix® is marketed and sold by Photocure in the Nordic countries and in the US with the trade name Cysview®. Photocure has a strategic partnership with Ipsen for the commercialization of Hexvix in Europe, excluding the Nordic region. Please refer to http://bit.ly/PHO-Partnering for further information on our commercial partners.
About Photocure ASA
Photocure, The Bladder Cancer Company, delivers transformative solutions to improve the lives of bladder cancer patients. Our unique technology, which makes cancer cells glow bright pink, has led to better health outcomes for patients worldwide. Photocure is headquartered in Oslo, Norway, and listed on the Oslo Stock Exchange (OSE: PHO). The US headquarters for Photocure Inc., are in Princeton, New Jersey. For more information, please visit us at www.photocure.com, www.hexvix.com or www.cysview.com
About KARL STORZ Endoscopy-America, Inc.
KARL STORZ Endoscopy-America, Inc., is an affiliate of KARL STORZ GmbH & Co. KG, an international leader for more than 70 years in reusable endoscope technology, encompassing all endoscopic specialties. Based in Tuttlingen, Germany, KARL STORZ GmbH & Co. KG is a family-owned company that designs, engineers, manufactures, and markets all its products with an emphasis on visionary design, precision craftsmanship and clinical effectiveness. For more information, call (800) 421-0837 or visit the company's website at www.karlstorz.com.
For more information, please contact:
Company contacts:
Kjetil Hestdal
President and CEO
Tel: +47 913 19 535
Email: kh@photcure.no
US President:
Ambaw Bellete
President
Photocure Inc.
Tel: + 1-609-759-6515
Email: ab@photocure.com
Media Relations:
Amanda Hudson
MCS Healthcare Public Relations
Tel: +1 908 234 9900
Email: amandah@mcspr.com
References:
1. SEER Cancer Statistics Factsheets: Bladder Cancer. National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed April 2018.
2. Bladder Cancer. American Cancer Society. http://www.cancer.org/acs/groups/cid/documents/webcontent/003085-pdf.pdf. Accessed April 2018.
3. Hall M, Chang S, Dalbagni G et al. Guideline for the Management of Nonmuscle Invasive Bladder Cancer (Stages Ta, T1, and Tis): 2007 Update. J Urol. 2007;178 (6):2314-2330.
4. Avritscher EB et al., Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006; 68:549-553.
5. Botteman et al. Clinical model of lifetime costs of treating bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003; 21:315-1330.
![]() View original content with multimedia:http://www.prnewswire.com/news-releases/photocure-announces-blue-light-cystoscopy-with-cysview-now-available-for-surveillance-of-bladder-cancer-300649109.html
View original content with multimedia:http://www.prnewswire.com/news-releases/photocure-announces-blue-light-cystoscopy-with-cysview-now-available-for-surveillance-of-bladder-cancer-300649109.html
SOURCE Photocure
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Intrexon Corpmehr Nachrichten
| Keine Nachrichten verfügbar. |