|
16.10.2017 07:30:00
|
VALBIOTIS: LIPIDRIVE®: Efficacy Confirmed in Three Models, Finalization of Recruitment for Phase I/II in Humans
Regulatory News:
This press release features multimedia. View the full release here: http://www.businesswire.com/news/home/20171015005016/en/
(Graphic: VALBIOTIS)
VALBIOTIS (Paris:ALVAL) (FR0013254851 - ALVAL / PEA/SME eligible), specialist in the development of innovative nutritional solutions for preventing cardiometabolic diseases and the provision of nutritional support for patients, has confirmed the efficacy of LIPIDRIVE® (LpD64) on weight gain and body fat mass in three preclinical models. These results were presented officially to the scientific community at the Cell Symposium: Metabolic Disease Therapies1 held in San Diego (USA) on 15 October 2017. They validate the proof of concept of the LpD64 candidate for preventing overweight and obesity.
Obesity has now spread beyond developed countries and is considered a global epidemic by the World Health Organization. It causes the death of some 2.8 million people a year worldwide2. This chronic condition has a serious impact on the overall health of those affected, contributing for example to the development of type 2 diabetes, the global prevalence of which is constantly increasing.
To prevent obesity and overcome this public health challenge, VALBIOTIS has developed LIPIDRIVE® (LpD64), a patented extract obtained from a combination of plants that aims to significantly reduce body fat mass and body weight.
> Presentation of LpD64 preclinical results: strong potential for preventing overweight and obesity
The Cell Symposia, organized in the United States by scientific media group Cell Press, publisher of the prestigious Cell journal, are world-famous conferences held several times a year that address cutting-edge topics and attract a large number of international scientists. A not-to-be-missed event for experts in metabolic diseases, the Cell Symposium: Metabolic Disease Therapies held in San Diego was dedicated to innovative therapeutic approaches for treating diabetes, obesity, NASH and their cardiovascular complications.
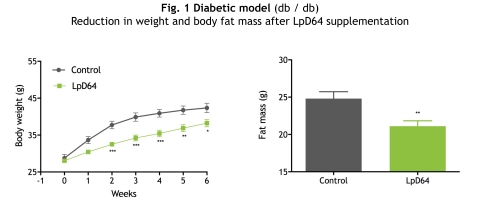
The preclinical results presented by VALBIOTIS in San Diego validated the efficacy of LpD64 on three mouse models of obesity and of metabolic diseases: a diabetic model (db/db), an obese model (ob/ob) and a model with diet-induced obesity (DIO).
The mouse models received a diet supplemented with LpD64 (2.7% of the diet) or a "control” diet for either 6 weeks (ob/ob and db/db models) or 16 weeks (DIO model).
In all models, LpD64 significantly reduced weight gain and body fat mass, without altering food intake and without affecting lean mass (fig. 1, 2 and 3).
The most significant effect was observed in models receiving a High-Fat Diet (HFD): LpD64 supplementation reduced body weight by 21% and body fat mass by 42% after 16 weeks compared to the mouse models receiving the same diet, without supplementation (fig. 3).
These very promising results demonstrate the efficacy of LpD64 on lipid metabolism and validate the proof of concept of LpD64. Significant beneficial effects can now legitimately be expected in humans, as part of a supplementation preventing overweight and obesity.
> Mechanism of action of LpD64: preliminary data and ongoing investigations
The mechanism by which LpD64 affects weight and body fat mass has been researched in several experiments already. Preliminary data on the gene expression of lipid metabolism markers in the liver and adipose tissue were presented in San Diego. At the same time, VALBIOTIS is carrying out research to determine the impact of LpD64 on intestinal microbiota (screening and sequencing of microbiota before and after supplementation), thought to contribute to the observed beneficial effects.
> Confirmation of tolerance in humans: finalization of recruitment for the ongoing
Phase I/II
The clinical development of LpD64 will begin with a Phase I/II study, which has already begun in humans3. This monocentric, controlled, open study aims to demonstrate the good tolerance of LpD64 by monitoring biological blood, urinary and hemodynamic parameters. VALBIOTIS is in the process of recruiting 20 obese male volunteers for the study, 18 of which have already been included.
"Our innovative Nutrition Healthcare approach seeks to develop solutions for preventing cardiometabolic diseases. By reducing body fat mass, a contributing and/or aggravating factor in high-risk conditions and cardiometabolic diseases, LpD64 clearly respects our health mission. The results confirmed in the preclinical models tested show the enormous potential of LpD64 in preventing overweight and obesity. Moving forward, our goal is to continue its clinical development in line with our high quality standards,” said Sébastien PELTIER, CEO of VALBIOTIS.
ABOUT VALBIOTIS
VALBIOTIS specializes in developing innovative nutrition solutions designed to prevent cardiometabolic diseases and provide nutritional support for patients. Its products are made for manufacturers in the agro-food and pharmaceutical industries. VALBIOTIS particularly focuses on solutions to prevent type 2 diabetes, NASH (nonalcoholic steatohepatitis), obesity and cardiovascular diseases.
VALBIOTIS was founded in La Rochelle in early 2014 and has formed numerous partnerships with top academic centers in France and abroad, including the La Rochelle University, the CNRS and the Clermont Auvergne University located in Clermont-Ferrand, where the company opened a second office. These partnerships have enabled VALBIOTIS to benefit from strong financial leverage, particularly thanks to experts and technical partners who support its projects. VALBIOTIS is a member of the "BPI Excellence” network and received the "Innovative Company” status accorded by BPI France. VALBIOTIS has also been awarded "Young Innovative Company” status and has received major financial support from the European Union for its research programs by obtaining support from the European Regional Development Fund (ERDF).
Find out more about VALBIOTIS:
http://valbiotis.com/en/
Name: VALBIOTIS
ISIN code: FR0013254851
Mnemonic code:
ALVAL
1
http://cell-symposia.com/metabolic-therapies-2017/
2
http://www.who.int/features/factfiles/obesity/en/
3
ClinicalTrials.gov: NCT0305206
View source version on businesswire.com: http://www.businesswire.com/news/home/20171015005016/en/
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Valbiotis SAmehr Nachrichten
| Keine Nachrichten verfügbar. |
Analysen zu Valbiotis SAmehr Analysen
Aktien in diesem Artikel
| Valbiotis SA | 1,27 | -2,15% |
|