|
03.04.2017 17:20:00
|
Versartis Announces Three Year Somavaratan Data at ENDO 2017 Showing Safety and Efficacy in Line with Historical U.S. Daily rhGH Data in Pediatric GHD
MENLO PARK, Calif., April 3, 2017 /PRNewswire/ -- Versartis, Inc. (NASDAQ:VSAR), an endocrine-focused biopharmaceutical company that is developing somavaratan, a novel, long-acting form of recombinant human growth hormone (rhGH) for growth hormone deficiency (GHD), announced that three-year safety and efficacy data on somavaratan in pediatric GHD were presented during an oral session at the Endocrine Society's 99th Annual Meeting & Expo (ENDO 2017), held April 1-4, 2017 in Orlando, Florida. The presentation will be made available at http://www.versartis.com/pipeline/publications/.
"The analysis of somavaratan following three years of treatment experience has shown it to be performing in line with daily rhGH data on key parameters, including height velocity, bone maturation and other key measures of catch-up growth, as well as IGF-I response, metabolic parameters, and safety," said Bradley S. Miller, M.D., Ph.D., Associate Professor in the Department of Pediatric Endocrinology at the University of Minnesota Masonic Children's Hospital. "These results reinforce the 3.5 mg/kg twice-monthly dose selected for the ongoing Phase 3 VELOCITY trial, with mean peak IGF-I SDS at this dose in the upper half of the normal therapeutic range and no unexpected safety concerns. It is highly encouraging that the three-year results are in line with results from U.S. registries, and this represents an incremental advancement of our knowledge of long-term treatment with somavaratan."
"The Versartis team's level of experience in the area of growth hormone is virtually unmatched, and we are all excited about the breadth and strength of our somavaratan data as we head into our pediatric Phase 3 results this fall," said Jay Shepard, President and CEO of Versartis, Inc. "We believe that somavaratan has the potential to be best-in-class for treating children with GHD based on the profile that we are building. Our aim with somavaratan is to provide the convenience of twice monthly dosing without any 'trade offs' to daily rhGh therapy, and our three-year data have shown safety and efficacy that are comparable. At the time of our anticipated launch, we expect to have safety data from up to five years of treatment as well as data on children who were switched from daily therapy, and we intend to price at parity. Our goal is to make somavaratan therapy an easy choice for physicians and their patients."
Presentation Summary and Data
31135: Safety and Efficacy of Somavaratan (VRS-317), a Long-Acting Recombinant Human Growth Hormone (rhGH), in Children with Growth Hormone Deficiency (GHD): 3-Year Update of the VERTICAL & VISTA Trials (NCT01718041, NCT02068521)
The presentation by Dr. Miller summarized safety and efficacy data for the 48 patients who completed three years of somavaratan treatment in the Phase 2a VERTICAL study and subsequent VISTA long-term safety study. Overall, the three-year VERTICAL/VISTA study results support the selection of 3.5 mg/kg twice-monthly dose regimen for the Phase 3 VELOCITY trial underway. Mean height velocity during Years 1, 2, and 3 following dose increase at the beginning of Year 2 was consistent across three years of treatment and with reported HV for daily rhGH therapy in the U.S. (see Figure 1 below). Height SDS for somavaratan during the three-year treatment period continued to improve from baseline and was in line with US registry data (Figure 2). Bone age versus chronologic age (Figure 3) were consistent with observed changes in U.S. registry studies. Furthermore, mean increase in bone age exceeded years on study, supporting catch-up growth. IGF-I responses with somavaratan achieved the target therapeutic range and were similar to those reported in U.S. registry data (Figure 4).
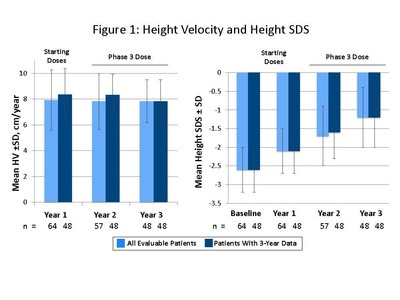
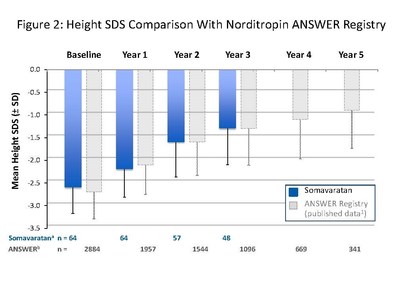

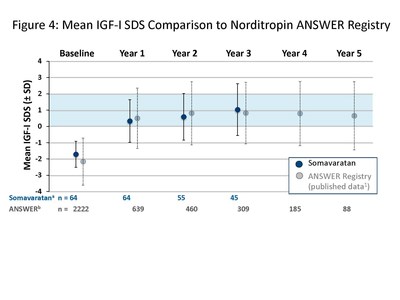
The safety profile remained consistent throughout three years of somavaratan treatment, with treatment-related adverse events that were generally mild and transient and no related serious adverse events. Metabolic parameters were normal and in line with daily rhGH.
About Somavaratan
Somavaratan is Versartis' investigational, novel, long-acting form of recombinant human growth hormone (rhGH). This fusion protein consists of rhGH and specific sequences of hydrophilic amino acids based on a proprietary XTEN® technology1. Somavaratan has been designed with the goal of improving therapeutic outcomes for children and adults with growth hormone deficiency (GHD), including enhanced adherence and convenience with a twice-monthly dosing schedule, fine gauge needle autoinjector device and room temperature storage.
Somavaratan is currently being evaluated for the treatment of pediatric GHD in the pivotal Phase 3 VELOCITY trial in the U.S., Canada and Europe, for which data are anticipated in September 2017, and the J14VR5 Phase 2/3 trial in Japan. Safety and efficacy data from 36 months of dosing in the Phase 2 trial and VISTA long-term safety study were presented during the Endocrine Society 2017 annual meeting. In adult GHD, results have been reported from the Phase 2 VITAL trial in the U.S., Europe and Australia and a Phase 3 trial is expected to begin by the end of 2017.
1XTEN is a registered trademark of Amunix Operating Inc.
About Versartis, Inc.
Versartis, Inc. is an endocrine-focused biopharmaceutical company initially developing somavaratan, a novel, long-acting form of recombinant human growth hormone in late-stage clinical trials for the treatment of GHD in children and adults. Somavaratan is intended to reduce the burden of daily injection therapy by requiring significantly fewer injections, potentially improving adherence and, therefore, treatment outcomes.
For more information on Versartis, visit www.versartis.com.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding our intentions or current expectations concerning, among other things, plans and timing of our clinical trials and the potential for eventual regulatory approval of somavaratan. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: our success being heavily dependent on somavaratan; somavaratan being a new molecular entity; the risk that somavaratan may not have favorable results in clinical trials or receive regulatory approval; potential delays in our clinical trials due to regulatory requirements or difficulty identifying qualified investigators or enrolling patients; the risk that somavaratan may cause serious side effects or have properties that delay or prevent regulatory approval or limit its commercial potential; the risk that we may encounter difficulties in manufacturing somavaratan; if somavaratan is approved, risks associated with its market acceptance, including pricing and reimbursement; potential difficulties enforcing our intellectual property rights; our reliance on our license of intellectual property from Amunix Operating, Inc. and our need for additional funds to support our operations. We discuss many of these risks in greater detail under the heading "Risk Factors" contained in our Annual Report on Form 10-K for the year ended December 31, 2016, which is on file with the Securities and Exchange Commission (SEC). Forward-looking statements are not guarantees of future performance, and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, may differ materially from the forward-looking statements contained in this press release. Any forward-looking statements that we make in this press release speak only as of the date of this press release. We assume no obligation to update our forward- looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
Contacts:
Corporate Communications:
Christine Labaree
Corporate Affairs
(650) 600-1697
clabaree@versartis.com
Investors:
David Burke
Director, Investor Relations
(650) 516-2703
dburke@versartis.com
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/versartis-announces-three-year-somavaratan-data-at-endo-2017-showing-safety-and-efficacy-in-line-with-historical-us-daily-rhgh-data-in-pediatric-ghd-300433427.html
SOURCE Versartis, Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Versartis Incmehr Nachrichten
| Keine Nachrichten verfügbar. |