|
25.10.2019 23:22:00
|
Vita Health Products Issues Voluntary Recall of Its Ranitidine Products in Canada
WINNIPEG, Manitoba, Oct. 25, 2019 /CNW/ -- As a precautionary measure, today Vita Health Products Inc. (the "Company") announced a voluntary Type I recall of all the Company's ranitidine products in Canada. This is due to the low level presence of N-nitrosodimethylamine (NDMA), an environmental contaminant and potential carcinogen found in water and foods, including meats, dairy products, and vegetables.

The bulk active ingredient used in Vita Health's ranitidine products is sourced from a third party. Upon initial testing of these products, Health Canada did find low levels of NDMA. As a result, and per direction from the supplier, Vita Health made the decision to conduct a voluntary recall. Although Vita Health has not received any complaints or adverse events associated with these products, this action is consistent with the Company's commitment to consumer safety.
In September 2019, The U.S. Food and Drug Administration, Health Canada and European regulators alerted manufacturers that, as a result of third party testing, NDMA had been detected in some generic and brand versions of ranitidine. They requested all manufacturers voluntarily stop shipping and complete further testing on ranitidine products, which Vita Health immediately complied with.
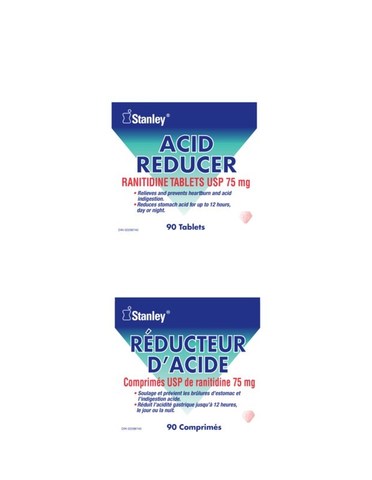
The below products are impacted by this recall:
Item | Product Description | UPC | Lots | ||||
307118 | STANLEY ACID REDUCER RANITIDINE 75MG 90 | 0 66000 02035 6 | All lots | ||||
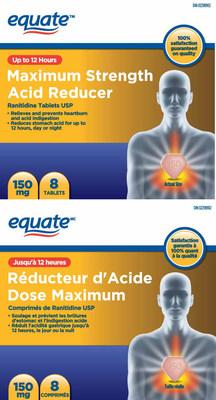
306939 | EQUATE MAXIMUM STRENGTH ACID REDUCER | 0 67002 30284 6 | All lots | ||||
306940 | EQUATE MAXIMUM STRENGTH ACID REDUCER | 0 67002 30285 3 | All lots | ||||
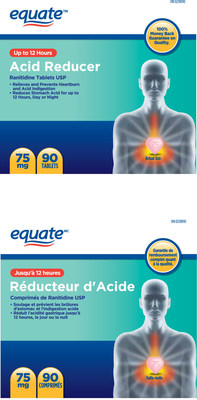
306170 | EQUATE ACID REDUCER RANITIDINE 75MG 10 | 0 67002 04685 6 | All lots | ||||
306175 | EQUATE ACID REDUCER RANITIDINE 75MG 30 | 0 67002 04686 3 | All lots | ||||
306178 | EQUATE ACID REDUCER RANITIDINE 75MG 60 | 0 66000 02055 4 | All lots | ||||
306180 | EQUATE ACID REDUCER RANITIDINE 75MG 90 | 0 67002 30001 9 | All lots | ||||
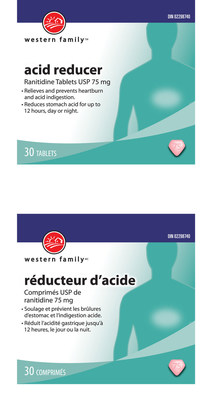
307317 | WESTERN FAMILY MAXIMUM STRENGTH ACID | 0 62639 32560 2 | All lots | ||||
307116 | WESTERN FAMILY ACID REDUCER RANITIDINE 75MG | 0 62639 24151 3 | All lots | ||||
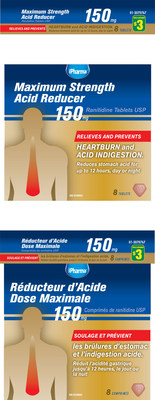
400182 | iPHARMA MAXIMUM STRENGTH ACID REDUCER | 6 67888 33789 7 | All lots | ||||
308286 | iPHARMA ACID REDUCER RANITIDINE 75MG | 0 67888 13248 3 | All lots | ||||
Consumers who have these products in their possession should return them to the place of purchase or call the Vita Health Customer Contact Centre at 1-877-637-7557, Monday-Friday 9 am-5:30 pm ET for further instruction.
For further information:
MEDIA CONTACT:
Nicole Hayes
Senior Manager, Corporate Communications
nhayes@nbty.com
1-631-200-2650

SOURCE Vita Health Products Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!